We are aware of the importance of magnesium as one of the three life essentials, together with oxygen and water, and as key role player in most enzymatic processes. We also know that on average there is about 28 grams of magnesium in our body, roughly divided 50/50 over teeth and bones one side and over blood and tissues on the other. But it’s a mistake to think that we can use the magnesium incorporated in teeth, bones and tissues as a buffer for poor times that can be refilled later.
Instead, like water and oxygen, we need a high turnover of magnesium and actually on a significant higher equilibrium than most of us have now.
Happily I met Julia; she made a nice animation to explain:
Animation by Julia Augusta Leal.
Magnesium, Magnesium Deficiency and Magnesium Supplementation:
Bosc fights against one of the most dangerous drugs: "Stop with Methylphenidate for children with ADHD, they need Magnesium in stead!"
Read about the books here:
Because there is as much evidence as discussion about transdermal effect of magnesium chloride and because academic research is lacking, the Magnesium Health Institute aims for scientifically sound research on the effects of dermally administrated magnesium oil (magnesium chloride / MgCl2 ). We developed a 3 step theoretical model that could explain supposed transdermal resorption.
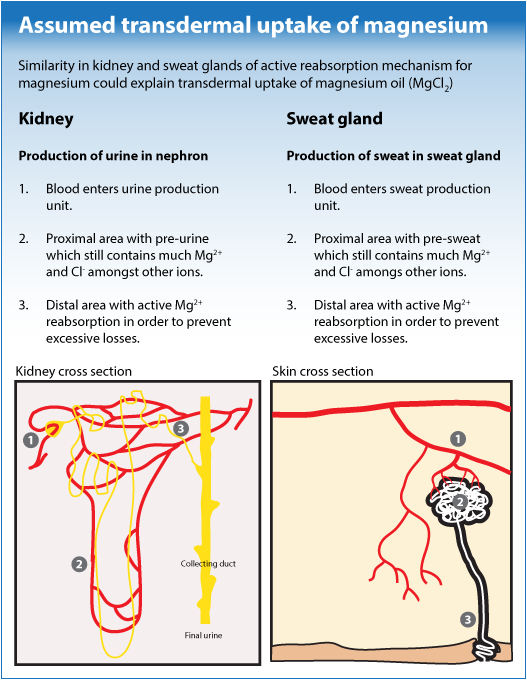
It is known that during urine production, something special is going on in our kidneys with magnesium: like many other ions, magnesium is abundantly excreted initially during urine production. In the further course of urine output many valuable components are regained. But in the so called distal tubules of the nephrons, just before final urine output, there are cells specially dedicated to actively re-absorb Mg. Not only by osmosis, diffusion, e-potential, or other non-energy consuming processes; no, those cells really actively reabsorb magnesium all the way through their membranes and cell content. It takes energy, but they just do it in order to protect us for magnesium losses!
Another way to lose minerals, including valuable magnesium, is sweating. Because sweat production has similarities with urine production, it would not be surprising if mother nature provided sweat glands with a somehow similar magnesium reabsorption mechanism. Actually there is growing scientific evidence for this assumption.
Magnesium oil applied on the skin or magnesium chloride in a bath brings magnesium ions into the distal part of sweat glands. When the ‘actively re-absorbing magnesium-cells’ detect a high concentration of magnesium, they will just do their job and (re-)absorb magnesium; even if it’s coming from outside: Transdermal uptake!
Please let us know what you think about this subject, leave us a message here.

For downloading the presentation by Richard Danel, MD (in .PDF) about the 14th International Magnesium Conference in Rome (June 2016):

The French physician Prof. Dr. Pierre Delbet, was one of the first true Magnesiologists.
He discovered the benifits of Magnesium Chloride (MgCl2) (1915).
Peruse his essay here:
In our body, the balance of two key minerals, calcium and magnesium have a significant impact on our health and quality of life. Inside the cells there is a lot of magnesium helping to keep calcium outside the cells. What happens if there is not enough magnesium? Here is a video by Andrea Rosanoff, PhD, Directing Scholar of the Center for Magnesium Education & Research.